Which Animal Bite May Pose A Risk For Rabies?
| Leptospirosis | |
|---|---|
| Other names | Rat fever,[1] field fever,[2] rat catcher's yellows,[3] pretibial fever[4] |
 | |
| Leptospira magnified 200-fold with a nighttime-field microscope. | |
| Specialty | Communicable diseases |
| Symptoms | None, headaches, muscle pains, fevers[five] |
| Complications | Bleeding from the lungs, meningitis, kidney failure[5] [6] |
| Usual onset | One to two weeks[7] |
| Causes | Leptospira typically spread past rodents[viii] |
| Risk factors | Exposure to infected animals or contaminated h2o[8] |
| Diagnostic method | Testing blood for antibodies against the bacterium or its Deoxyribonucleic acid[5] |
| Differential diagnosis | Malaria, enteric fever, rickettsiosis, dengue[nine] |
| Prevention | Personal protective equipment, hygiene measures, doxycycline[7] |
| Treatment | Doxycycline, penicillin, ceftriaxone[8] |
| Prognosis | Risk of expiry ~7.5%[10] |
| Frequency | One one thousand thousand people per twelvemonth[vii] [eleven] |
| Deaths | 58,900 per yr[11] |
Leptospirosis is a claret infection acquired by the leaner Leptospira.[8] Signs and symptoms tin range from none to balmy (headaches, muscle pains, and fevers) to severe (bleeding in the lungs or meningitis).[five] Weil's disease, the acute, severe class of leptospirosis, causes the infected individual to become jaundiced (pare and optics become yellow), develop kidney failure, and bleed.[6] Bleeding from the lungs associated with leptospirosis is known as severe pulmonary haemorrhage syndrome.[five]
More x genetic types of Leptospira cause illness in humans.[12] Both wild and domestic animals can spread the illness, most unremarkably rodents.[8] The bacteria are spread to humans through fauna urine, or h2o and soil contaminated with animal urine, coming into contact with the eyes, oral fissure, nose or breaks in the skin.[eight] In developing countries, the disease occurs nearly commonly in farmers and depression-income people who live in areas with poor sanitation.[v] In adult countries, it occurs during heavy downpours and is a risk to sewage workers[13] and those involved in outdoor activities in warm and moisture areas.[v] Diagnosis is typically by testing for antibodies confronting the bacteria or finding bacterial Deoxyribonucleic acid in the blood.[5]
Efforts to foreclose the illness include protective equipment to cake contact when working with potentially infected animals, washing later contact, and reducing rodents in areas where people live and work.[7] The antibiotic doxycycline is effective in preventing leptospirosis infection.[7] Man vaccines are of limited usefulness;[fourteen] vaccines for other animals are more widely available.[15] Treatment when infected is with antibiotics such equally doxycycline, penicillin, or ceftriaxone.[8] The overall run a risk of death is 5–10%.[10] All the same, when the lungs are involved, the risk of death increases to the range of fifty–seventy%.[8]
It is estimated that one million severe cases of leptospirosis occur every year, causing well-nigh 58,900 deaths.[xi] The illness is about common in tropical areas of the earth merely may occur anywhere.[seven] Outbreaks may arise after heavy rainfall.[7] The illness was first described by physician Adolf Weil in 1886 in Germany.[16] [17]
Signs and symptoms [edit]
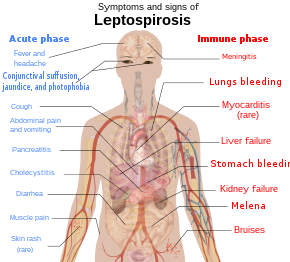
Schematic depiction of the symptoms and signs of leptospirosis.

The symptoms of leptospirosis usually appear 1 to 2 weeks later on infection,[7] only the incubation menstruation can be as long as a month.[18] The disease is biphasic in a majority of symptomatic cases. Symptoms of the first phase (acute or leptospiremic phase) last five to vii days. In the second phase (immune phase), the symptoms resolve equally antibodies against the leaner are produced.[8] Boosted symptoms develop in the second stage.[19] The phases of illness may non be distinct, especially in patients with astringent disease.[20] ninety% of those infected feel mild symptoms while ten% experience severe leptospirosis.[21]
Leptospiral infection in humans causes a range of symptoms, though some infected persons may have none. The disease begins all of a sudden with fever accompanied by chills, intense headache, severe musculus aches and intestinal pain.[5] [18] A headache brought on past leptospirosis causes throbbing hurting and is characteristically located at the head's bilateral temporal or frontal regions. The person could too have pain behind the optics and a sensitivity to light. Musculus pain commonly involves the calf musculus and the lower back. The nearly feature feature of leptospirosis is the conjunctival suffusion (conjunctivitis without exudate) which is rarely found in other febrile illnesses. Other characteristic findings on the eye include subconjunctival bleeding and jaundice. A rash is rarely found in leptospirosis. When ane is found alternative diagnoses such as dengue fever and chikungunya fever should exist considered. Dry cough is observed in 20–57% of people with leptospirosis. Thus, this clinical feature can mislead a medico to diagnose the disease equally a respiratory affliction. Additionally, gastrointestinal symptoms such as nausea, vomiting, intestinal pain, and diarrhoea frequently occur. Vomiting and diarrhea may contribute to dehydration. The abdominal hurting can be due to acalculous cholecystitis or inflammation of the pancreas.[18] Rarely, the lymph nodes, liver, and spleen may be enlarged and palpable.[8]
There will be a resolution of symptoms for i to iii days.[7] The allowed stage starts later this and tin can last from iv to 30 days and can be anything from brain to kidney complications.[22] The authentication of the second phase is inflammation of the membranes covering the brain.[7] Signs and symptoms of meningitis include severe headache and neck stiffness.[vii] Kidney involvement is associated with reduced or absent-minded urine output.[7]
The classic form of severe leptospirosis, known equally Weil'south disease, is characterised past liver damage (causing jaundice), kidney failure, and bleeding, which happens in 5–ten% of those infected.[7] Lung and brain damage can also occur. For those with signs of inflammation of membranes covering the brain and the brain itself, altered level of consciousness tin can happen. A variety of neurological issues such as paralysis of half of the torso, complete inflammation of a whole horizontal department of spinal cord, and musculus weakness due to immune harm of the nerves supplying the muscles are the complications. Signs of bleeding such equally non-traumatic bruises at i mm (0.039 in), not-traumatic bruises more than 1 cm (0.39 in), nose haemorrhage, blackish stools due to haemorrhage in the breadbasket, airsickness blood and bleeding from the lungs can also exist found. Prolongation of prothrombin fourth dimension in coagulation testing is associated with severe bleeding manifestation. However, low platelet count is not associated with severe haemorrhage.[18] Pulmonary bleeding is alveolar bleeding (bleeding into the alveoli of the lungs) leading to massive coughing upwardly of blood, and causing astute respiratory distress syndrome, where the run a risk of expiry is more than than 50%.[eighteen] Rarely, inflammation of the middle muscles, inflammation of membranes covering the heart, abnormalities in the heart's natural pacemaker and aberrant heart rhythms may occur.[eight]
Crusade [edit]
Leaner [edit]
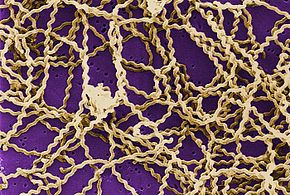
Leptospirosis is acquired by spirochaete bacteria that vest to the genus Leptospira, which are aerobic,[8] right-handed helical,[12] and 6 –xx micrometers long.[seven] Like Gram-negative leaner, Leptospira have an outer membrane studded with lipopolysaccharide (LPS) on the surface, an inner membrane and a layer of peptidoglycan cell wall. However, dissimilar Gram-negative bacteria, the peptidoglycan layer in Leptospira lies closer to the inner than the outer membrane. This results in a fluid outer membrane loosely associated with the cell wall.[23] In add-on, Leptospira have a flagellum located in the periplasm, associated with corkscrew mode movement.[seven] Chemoreceptors at the poles of the leaner sense various substrates and change the direction of its movement.[12] The bacteria are traditionally visualised using dark-field microscopy without staining.[vii]
A total of 66 species of Leptospira has been identified. Based on their genomic sequence, they are divided into two clades and four subclades: P1, P2, S1, and S2.[24] The 19 members of the P1 subclade include the 8 species that can cause severe disease in humans: L. alexanderi, Fifty. borgpetersenii, L. interrogans, 50. kirschneri, L. mayottensis, L. noguchii, L. santarosai, and Fifty. weilii. [12] [24] The P2 clade comprises 21 species that may cause mild disease in humans. The remaining 26 species comprise the S1 and S2 subclades, which include "saprophytes" known to consume decomposable matter (saprotrophic nutrition).[24] Pathogenic Leptospira practise not multiply in the environment. Leptospira crave loftier humidity for survival but tin can remain live in environments such equally stagnant h2o or contaminated soil. The bacterium can exist killed by temperatures of fifty °C (122 °F) and can be inactivated past 70% ethanol, 1% sodium hypochlorite, formaldehyde, detergents and acids.[25]
Leptospira are also classified based on their serovar. The diverse carbohydrate limerick of the lipopolysaccharide on the surface of the leaner is responsible for the antigenic difference between serovars.[12] Over 250 pathogenic serovars of Leptospira are recognised, with closely related serovars gathered into over 26 pathogenic serogroups.[8] Strains of dissimilar species of Leptospira may be members of the same serogroup because of horizontal factor transfer of LPS biosynthetic genes between unlike species.[12]
Manual [edit]
The leaner can be found in ponds, rivers, puddles, sewers, agricultural fields and moist soil.[seven] Pathogenic Leptospira take been plant in the form of aquatic biofilms, which may aid survival in the environment.[26]
Leptospira live in the kidneys of diverse wild and domestic animals. When animals ingest the bacteria, they circulate in the bloodstream, then lodge themselves into the kidneys through the glomerulular or peritubular capillaries. The bacteria then pass into the lumens of the renal tubules and colonise the brush border of the proximal convoluted tubule. This causes the continuous shedding of leaner in the urine without the animal experiencing significant ill furnishings. This relationship between the creature and the leaner is known as a commensal relationship, and the animate being is known every bit a reservoir host.[18]
Leptospira are institute mostly in mammals.[5] However, reptiles and cold-blooded animals such every bit frogs, snakes, turtles, and toads have been shown to take the infection.[15] Whether there are reservoirs of man infection is unknown.[18] [xv] Rats, mice, and moles are of import principal hosts, simply other mammals including dogs, deer, rabbits, hedgehogs, cows, sheep, swine, raccoons, opossums, and skunks can also acquit the affliction.[15] In Africa, a number of wildlife hosts have been identified as carriers, including the banded mongoose, Egyptian fox, Rusa deer, and shrews.[27] There are various mechanisms whereby animals can infect each other. Dogs may lick the urine of an infected fauna off the grass or soil, or beverage from an infected puddle.[ citation needed ] Firm-jump domestic dogs have contracted leptospirosis, patently from licking the urine of infected mice in the business firm.[ citation needed ] Leptospirosis can as well be transmitted via the semen of infected animals.[15] The duration of bacteria existence consistently present in animal urine may persist for years.[15]
Humans are the accidental host of Leptospira.[5] Humans get infected through contact with water or moist soil that contains urine from infected animals.[seven] The bacteria enter through cuts, abrasions,[7] ingestion of contaminated food, or contact with mucous membrane of the body (e.yard. oral cavity, nose, and eyes).[28] Occupations at take a chance of contracting leptospirosis include farmers, fishermen, garbage collectors and sewage workers.[v] The disease is likewise related to adventure tourism and recreational activities.[5] Information technology is common among h2o-sports enthusiasts in specific areas, including triathlons, water rafting, canoeing and swimming, as prolonged immersion in water promotes the entry of the bacteria.[5] However, Leptospira are unlikely to penetrate intact peel.[8] The disease is not known to spread between humans, and bacterial dissemination in recovery period is extremely rare in humans.[eight] In one case humans are infected, bacterial shedding from the kidneys usually persists for up to sixty days.[25]
Rarely, leptospirosis can be transmitted through an organ transplant.[29] Infection through the placenta during pregnancy is likewise possible.[30] [31] [32] It can cause miscarriage and infection in infants.[33] Leptospirosis transmission through eating raw meat of wild animals animals have also been reported (e.1000. psychiatric patients with allotriophagy).[34]
Pathogenesis [edit]

Means of Leptospira bacteria infecting human cells and blood stream.
The pathogenesis of leptospirosis remains poorly understood despite research efforts.[seven] [28] The leaner enter the human body through either breaches in the pare or the mucous membrane, then into the bloodstream. The leaner afterward attach to the endothelial cells of the blood vessels and extracellular matrix (complex network of proteins and carbohydrates nowadays between cells). The leaner use their flagella for moving between cell layers. They bind to cells such as fibroblasts, macrophages, endothelial cells, and kidney epithelial cells. They also bind to several human proteins such as complement proteins, thrombin, fibrinogen, and plasminogen using surface leptospiral immunoglobulin-like (Lig) proteins such every bit LigB and LipL32, whose genes are found in all pathogenic species.[12] [28]
Through innate immune system, endothelial cells of the capillaries in the human body are activated by the presence of these bacteria. The endothelial cells produce cytokines and antimicrobial peptides against the leaner. These products regulate the coagulation cascade and movements of white blood cells.[12] Macrophages presented in humans are able to engulf Leptospira. Notwithstanding, Leptospira are able to reside and proliferate in the cytoplasmic matrix later on being ingested past macrophages.[12] Those with astringent leptospirosis can experience a loftier level of cytokines such equally interleukin half-dozen, tumor necrosis cistron alpha (TNF-α), and interleukin x. The high level of cytokines causes sepsis-like symptoms which is life-threatening instead of helping to fight against the infection.[21] Those who have a high risk of sepsis during a leptospirosis infection are found to take the HLA-DQ6 genotype, possibly due to superantigen activation, which damages actual organs.[18]
Humoral immunity is the main immune response against the Leptospira cells. Agglutinating antibodies such as immunoglobulin Thou and immunoglobulin G are produced against the bacteria. Such antibodies are mainly directed against the LPS.[28] Leptospira LPS just activates toll-like receptor ii (TLR2) in monocytes in humans. The lipid A molecule of the bacteria is not recognised by human being TLR4 receptors. Therefore, the lack of Leptospira recognition by TLR4 receptors probably contributes to the leptospirosis illness procedure in humans.[12]
Although there are diverse mechanisms in the human being torso to fight against the bacteria, Leptospira is well adapted to such an inflammatory status created by information technology. In the bloodstream, it tin can activate host plasminogen to go plasmin that breaks downwards extracellular matrix, degrades fibrin clots and complemental proteins (C3b and C5) to avert opsonisation. It can likewise recruit complement regulators such equally Factor H, C4b-binding protein, factor H-similar binding poly peptide, and vitronectin to prevent the activation of membrane assault circuitous on its surface. Information technology as well secretes proteases to degrade complement proteins such as C3. Information technology tin bind to thrombin that decreases the fibrin formation. Reduced fibrin formation increases the risk of bleeding.[12] Leptospira too secretes sphingomyelinase and haemolysin that target reddish blood cells.[vii]
Leptospira spreads rapidly to all organs through the bloodstream.[12] They mainly affect the liver. They invade spaces between hepatocytes, causing apoptosis. The damaged hepatocytes and hepatocyte intercellular junctions cause leakage of bile into the bloodstream, causing elevated levels of bilirubin, resulting in jaundice. Congested liver sinusoids and perisinusoidal spaces have been reported. Meanwhile, in the lungs, petechiae or frank bleeding tin can exist institute at the alveolar septum and spaces between alveoli.[18] Leptospira secretes toxins that crusade mild to severe kidney failure or interstitial nephritis.[28] The kidney failure can recover completely or lead to cloudburst and fibrosis.[eighteen] Inflammation of the heart muscles, coronary arteries, and aorta is rare.[22]
Diagnosis [edit]

Kidney tissue, using a silverish staining technique, revealing the presence of Leptospira bacteria

Diffuse lungs haemorrhage due to leptospirosis infection.
Laboratory tests [edit]
For those who are infected, a consummate blood count may show a high white prison cell count and a depression platelet count. When a depression haemoglobin count is present together with a low white cell count and thrombocytopenia, os marrow suppression should be considered.[18] Erythrocyte sedimentation rate and C-reactive protein may besides be elevated.[8]
The kidneys are usually involved in leptospirosis. Claret urea and creatinine levels will be elevated. Leptospirosis increases potassium excretion in urine, which leads to a low potassium level[18] and a depression sodium level in the blood.[8] [18] Urinalysis may reveal the presence of protein, white blood cells, and microscopic haematuria.[viii] Because the leaner settle in the kidneys, urine cultures volition be positive for leptospirosis starting after the second week of affliction until 30 days of infection.[eight]
For those with liver involvement, transaminases and directly bilirubin are elevated in liver function tests. The Icterohaemorrhagiae serogroup is associated with jaundice and elevated bilirubin levels. Hemolytic anemia contributes to jaundice. A feature of leptospirosis is astute haemolytic anaemia and conjugated hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase deficiency.[18] Abnormal serum amylase and lipase levels (associated with pancreatitis) are constitute in those who are admitted to hospital due to leptospirosis. Dumb kidney part with creatinine clearance less than 50 ml/min is associated with elevated pancreatic enzymes.[18]
For those with astringent headache who show signs of meningitis, a lumbar puncture can be attempted. If infected, cerebrospinal fluid (CSF) examination shows lymphocytic predominance with a cell count of well-nigh 500/mm3, poly peptide between 50 and 100 mg/ml and normal glucose levels. These findings are consistent with hygienic meningitis.[18]
Serological tests [edit]
Rapid detection of Leptospira can exist done past quantifying the IgM antibodies using ELISA. Typically, 50. biflexa antigen is used to detect the IgM antibodies. This examination can quickly decide the diagnosis and help in early handling. Still, the test specificity depends upon the type of antigen used and the presence of antibodies from previous infections. The presence of other diseases such as Epstein-Barr virus infection, viral hepatitis, and cytomegalovirus infection can cause false-positive results.[18] Other rapid screening tests have been developed such as dipsticks, latex and slide agglutination tests.[viii]
The microscopic agglutination test (MAT) is the reference test for the diagnosis of leptospirosis.[18] MAT is a exam where serial dilutions of patient sera are mixed with different serovars of Leptospira. The mixture is then examined under a dark field microscope to await for agglutination. The highest dilution where l% agglutination occurs is the result.[xviii] MAT titres of 1:100 to one:800 are diagnostic of leptospirosis.[8] A fourfold or greater ascent in titre of 2 sera taken at symptoms' onset and three to ten days of affliction onset confirms the diagnosis. During the astute phase of the disease, MAT is not specific in detecting a serotype of Leptospira because of cantankerous-reactivity between the serovars.[18] In the convalescent phase, MAT is more specific in detecting the serovar types.[18] MAT requires a console of live antigens and requires laborious work.[22]
Molecular tests [edit]
Leptospira DNA tin exist amplified by using polymerase chain reaction (PCR) from serum, urine, aqueous humour, CSF, and dissection specimens.[18] PCR can observe Leptospira Deoxyribonucleic acid in claret even before the antibody response develops. Every bit PCR detects the presence of Leptospira DNA, it is useful even after antibiotic treatment has started.[8]
Imaging [edit]
In those who have lung interest, a chest Ten-ray may demonstrate diffuse alveolar opacities.[18]
Diagnostic criteria [edit]
In 1982, the World Health Organization (WHO) proposed the Faine's criteria for the diagnosis of leptospirosis. It consists of 3 parts: A (clinical findings), B (epidemiological factors), and C (lab findings and bacteriological data). Since the original Faine's criteria just included culture and MAT in part C, which is difficult and complex to perform, the modified Faine'due south criteria was proposed in 2004 to include ELISA and slide agglutination tests which are easier to perform. In 2012, modified Faine's criteria (with amendment) was proposed to include shortness of breath and cough up claret in the diagnosis. In 2013, India recommended modified Faine's criteria in the diagnosis of leptospirosis.[35]
Prevention [edit]

A observe board by a lakeside in Sarawak, Malaysia that warns against swimming in the lake as information technology has tested positive for pathogenic Leptospira.

Blood samples beingness taken from a group of residents in Boyolali Regency, Indonesia for leptospirosis screening tests.
Rates of leptospirosis tin can be reduced by improving housing, infrastructure, and sanitation standards. Rodent abatement efforts and flood mitigation projects tin besides assist to foreclose it.[18] Proper use of personal protective equipment (PPE) by people who have a high risk of occupational exposure tin can preclude leptospirosis infections in nearly cases.[eighteen]
There is no human vaccine suitable for worldwide use.[fourteen] Just Cuba, Japan, France, and Cathay take approved the use of leptospirosis vaccines, and they are administered only to those in high-risk occupations and in response to floods and epidemics.[18] [14] [36] The vaccines are composed of killed Leptospira, and they confer immunity only to the serovar independent in the vaccine.[36] Side effects such as nausea, injection site redness and swelling have been reported later on the vaccine was injected. Since the immunity induced past one Leptospira serovar is only protective against that specific ane, trivalent vaccines have been adult.[18] Amnesty post-obit vaccination lasts for about a year.[36]
Doxycycline is given in one case a calendar week as a prophylaxis and is effective in reducing the rate of leptospirosis infections amongst loftier-take a chance individuals in flood-decumbent areas.[37] In one written report, it reduced the number of leptospirosis cases in military personnel undergoing exercises in the jungles. In another study, it reduced the number of symptomatic cases subsequently exposure to leptospirosis under heavy rainfall in endemic areas.[18]
Treatment [edit]
Most leptospiral cases resolve spontaneously. Early initiation of antibiotics may foreclose the progression to severe disease. Therefore, in resources-limited settings, antibiotics can be started in one case leptospirosis is suspected after history taking and exam.[18]
For mild leptospirosis, antibiotic recommendations such as doxycycline, azithromycin, ampicillin and amoxicillin were based solely on in vitro testing.[8] In 2001, the WHO recommended oral doxycycline (2 mg/kg up to 100 mg every 12 hours) for v to seven days for those with mild leptospirosis. Tetracycline, ampicillin, and amoxicillin can likewise be used in such cases.[38] However, in areas where rickettsia and leptospirosis are both endemic, azithromycin and doxycycline are the drugs of choice.[8]
Based on a 1988 study, intravenous (Iv) benzylpenicillin (besides known as penicillin Thousand) is recommended for the treatment of severe leptospirosis.[8] Intravenous benzylpenicillin (30 mg/kg up to ane.2 m every six hours) is used for five to seven days. Amoxicillin, ampicillin, and erythromycin may too be used for severe cases.[38] Ceftriaxone (i g IV every 24 hours for seven days) is also effective for severe leptospirosis.[18] [8] [39] Cefotaxime (1 g IV every six hours for seven days) and doxycycline (200 mg initially followed by 100 mg Iv every 12 hours for seven days) are as effective equally benzylpenicillin (1.5 one thousand thousand units 4 every six hours for seven days).[8] [40] Therefore, there is no evidence on differences in death reduction when benzylpenicillin is compared with ceftriaxone or cefotaxime.[8] Another study conducted in 2007 also showed no difference in efficacy between doxycycline (200 mg initially followed past 100 mg orally every 12 hours for 7 days) or azithromycin (2 thou on twenty-four hours one followed past 1 g daily for ii more than days) for suspected leptospirosis. There was no departure in the resolution of fever and azithromycin is better tolerated than doxycycline.[41] [42] [43]
Outpatients are given doxycycline or azithromycin. Doxycycline can shorten the duration of leptospirosis by 2 days, ameliorate symptoms, and prevent the shedding of organisms in their urine. Azithromycin and amoxicillin are given to pregnant women and children.[xviii] Rarely, a Jarisch–Herxheimer reaction can develop in the first few hours later on antibiotic administration.[8] However, according to a meta-analysis done in 2012, the do good of antibiotics in the handling of leptospirosis was unclear although the use of antibiotics may reduce the duration of illness by ii to four days.[8] [42] Another meta-analysis washed in 2013 reached a similar conclusion.[8] [43]
For those with severe leptospirosis, including potassium wasting with high kidney output dysfunction, intravenous hydration and potassium supplements can prevent dehydration and hypokalemia. When acute kidney failure occurs, early initiation of haemodialysis or peritoneal dialysis can help to improve survival. For those with respiratory failure, tracheal intubation with low tidal volume improves survival rates.[18]
Corticosteroids have been proposed to suppress inflammation in leptospirosis considering Leptospira infection can induce the release of chemical signals which promote inflammation of blood vessels in the lungs. However, there is bereft evidence to determine whether the use of corticosteroids is beneficial.[8] [44]
Prognosis [edit]
The overall risk of death for leptospirosis is 5–ten%.[x] For those with jaundice, the case fatality can increase up to 15%.[25] For those infected who present with confusion and neurological signs, there is a loftier risk of death.[18] Other factors that increase the hazard of decease include reduced urine output, age more than 36 years, and respiratory failure.[18] With proper intendance, well-nigh of those infected will recover completely. Those with acute kidney failure may suffer persistent balmy kidney impairment later on they recover.[18] In those with severe lung involvement, the risk of decease is 50–70%.[8]
In 1 study, 30% of patients who recovered from acute leptospirosis complained of long-lasting fatigue, malaise, weakness, muscle pain, and headaches. In 21% of these patients, these symptoms lasted for more than 2 years.[18] Centre problems occur in 10% of those who recovered from leptospirosis.[25] These complications range from mild anterior uveitis to severe panuveitis (which involves all three vascular layers of the eye) post-recovery. In upwards to fourscore% of those infected, Leptospira Dna is detected in the aqueous humour of the eye.[eighteen] Eye bug unremarkably have a skilful prognosis following treatment or they are self-limiting.[25]
Epidemiology [edit]
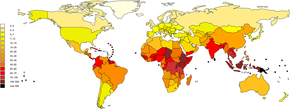
Information technology is estimated that one meg astringent cases of leptospirosis occur annually, with 58,900 deaths. Severe cases business relationship for 5-xv% of all leptospirosis cases.[eleven] Leptospirosis is found in both urban and rural areas in tropical, subtropical, and temperate regions.[x] The global health burden for leptospirosis can be measured by inability-adjusted life year (DALY). The score is 42 per 100,000 people per year, which is more than other diseases such as rabies and filariasis.[7]
The disease is observed persistently in parts of Asia, Oceania, the Caribbean, Latin America and Africa.[25] Antarctica is the only place not affected by leptospirosis.[25] In the Usa, there were 100 to 150 leptospirosis cases annually.[45] In 1994, leptospirosis ceased to exist a notifiable illness in the United states except in 36 states/territories where it is prevalent such equally Hawaii, Texas, California, and Puerto Rico.[46] About 50% of the reported cases occurred in Puerto Rico. In Jan 2013, leptospirosis was reinstated as a nationally notifiable disease in the United States.[45] Research on epidemiology of leptospirosis in high-take chances groups and risk factors is express in India.[47]
The global rates of leptospirosis take been underestimated because most afflicted countries lack notification or notification is not mandatory.[18] Distinguishing clinical signs of leptospirosis from other diseases and lack of laboratory diagnostic services are other problems.[48] The socioeconomic status of many of the earth's population is closely tied to malnutrition; subsequent lack of micronutrients may atomic number 82 to increased risk of infection and decease due to leptospirosis infection.[49] Micronutrients such equally iron, calcium, and magnesium represent important areas for time to come inquiry.[49]

Working in a paddy field barefoot is a chance gene for leptospirosis.
The number of cases of leptospirosis is directly related to the amount of rainfall, making the illness seasonal in temperate climates and yr-round in tropical climates.[7] The risk of contracting leptospirosis depends upon the risk of disease carriage in the community and the frequency of exposure.[xviii] In rural areas, farming and creature husbandry are the major risk factors for contracting leptospirosis.[5] Poor housing and inadequate sanitation as well increase the risk of infection.[18] In tropical and semi-tropical areas, the disease oft becomes widespread after heavy rains or afterwards flooding.[7]
History [edit]
The affliction was first described by Adolf Weil in 1886 when he reported an "acute communicable diseases with enlargement of spleen, jaundice, and nephritis."[17] Before Weil's description, the disease was known as "rice field jaundice" in ancient Chinese text, "autumn fever", "seven-day fever",[50] and "nanukayami fever"[51] in Japan; in Europe and Commonwealth of australia, the disease was associated with certain occupations and given names such as "cane-cutter's affliction", "swine-herd'southward disease", and "Schlammfieber" (mud fever).[fifty] It has been known historically equally "black jaundice",[52] or "dairy farm fever" in New Zealand.[53] Leptospirosis was postulated as the cause of an epidemic among Native Americans along the coast of what is now New England during 1616–19. The affliction was most likely brought to the New World by Europeans.[54]
Leptospira was beginning observed in 1907 in a post mortem kidney tissue slice by Arthur Stimson using silvery degradation staining technique. He called the organism Spirocheta interrogans considering the bacteria resembled a question marking.[fifty] [55] In 1908, a Japanese research group led by Ryukichi Inada and Yutaka Ito get-go identified this bacterium every bit the causative agent of leptospirosis[56] and noted its presence in rats in 1916.[57] Japanese coal mine workers frequently contracted leptospirosis. In Nihon, the organism was named Spirocheta icterohaemorrhagiae. The Japanese group likewise experimented with the kickoff leptospiral immunisation studies in guinea pigs. They demonstrated that by injecting the infected republic of guinea pigs with sera from convalescent humans or goats, passive amnesty could be provided to the republic of guinea pigs. In 1917, the Japanese group discovered rats as the carriers of leptospirosis.[l] Unaware of the Japanese group's work, two German language groups independently and almost simultaneously published their first demonstration of transmitting leptospiral infection in guinea pigs in October 1915. They named the organism Spirochaeta nodosa and Spirochaeta Icterogenes respectively.[fifty]
Leptospirosis was subsequently recognised as a disease of all mammalian species. In 1933, Dutch workers reported the isolation of Leptospira canicola which specifically infects dogs. In 1940, the strain that specifically infects cattle was first reported in Russia.[50] In 1942, soldiers at Fort Bragg, North Carolina, were recorded to have an infectious disease which caused a rash over their shinbones. This disease was later known to be caused by leptospirosis.[18] Past the 1950s, the number of serovars that infected various mammals had expanded significantly. In the 1980s, leptospirosis was recognised as a veterinary disease of major economic importance.[50]
In 1982, at that place were about 200 serovars of Leptospira available for nomenclature. The International Committee on Systematic Bacteriology's subcommittee on taxonomy of Leptospira proposed classifying these serovars into two big groups: Fifty. interrogans containing pathogenic serovars and L. biflexa containing saprophytic serovars.[l] In 1979, the leptospiral family of Leptospiraceae was proposed. In the aforementioned year, Leptospira illini was reclassified as the new genus Leptonema.[l] In 2002, "Lepthangamushi syndrome" was coined to describe a series of overlapping symptoms of leptospirosis with Hantavirus hemorrhagic fever with renal syndrome, and scrub typhus acquired by Orientia tsutsugamushi.[58] [59] In 2005, Leptospira parva was classified as Turneriella.[50] With DNA-Dna hybridisation engineering, L. interrogans was divided into 7 species. More than Leptospira species have been discovered since so.[50] The WHO established the Leptospirosis Burden Epidemiology Reference Group (LERG) to review the latest disease epidemiological data of leptospirosis, formulate a affliction manual model, and identify gaps in knowledge and research. The first meeting was convened in 2009. In 2011, LERG estimated that the global yearly rate of leptospirosis is five to 14 cases per 100,000 population.[18]
Other animals [edit]
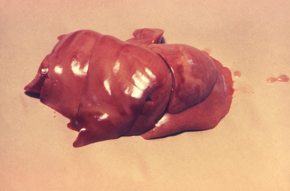
Liver of an unknown animal with multiple blackish necrotic patches secondary to leptospirosis infection.

Lungs of a canine with multiple bleeding spots due to leptospirosis.
Infected animals can have no, mild, or severe symptoms;[60] the presenting symptoms may vary past the blazon of animal.[15] [threescore] In some animals the bacteria live in the reproductive tract, leading to manual during mating.[15]
Animals besides present with similar clinical features when compared to humans. Clinical signs tin appear in 5–xv days in dogs. The incubation catamenia can be prolonged in cats. Leptospirosis tin cause abortions later ii–12 weeks in cattle, and ane–4 weeks of infection in pigs. The illness tends to exist milder in reservoir hosts. The most commonly affected organs are the kidneys, liver, and reproductive organization, but other organs can be affected.[25] In dogs, the astute clinical signs include fever, loss of ambition, shivering, musculus hurting, weakness, and urinary symptoms. Airsickness, diarrhea, and intestinal pain may also nowadays. Petechiae and ecchymoses may be seen on mucous membranes. Bleeding from the lungs may likewise be seen in dogs. In chronic presentations, the affected dog may have no symptoms. In animals that have died of leptospirosis, their kidneys may be bloated with grey and white spots, mottling, or scarring. Their liver may be enlarged with areas of cell death. Petechiae and ecchymoses may exist found in various organs.[25] [61] Inflammation of the claret vessels, inflammation of the heart, meningeal layers covering the brain and spinal cord, and uveitis are also possible.[15] Equine recurrent uveitis (ERU) is the most mutual disease associated with Leptospira infection in horses in Due north America and may lead to incomprehension.[62] [63] ERU is an autoimmune illness involving antibodies confronting Leptospira proteins LruA and LruB cross-reacting with eye proteins.[62] Live Leptospira can be recovered from the aqueous or vitreous fluid of many horses with Leptospira-associated ERU.[63] Risk of expiry or disability in infected animals varies depending upon the species and age of the animals. In adult pigs and cattle, reproductive signs are the most mutual signs of leptospirosis. Up to 40% of cows may have a spontaneous ballgame. Younger animals usually develop more severe disease. Nigh eighty% of dogs can survive with treatment, but the survival rate is reduced if the lungs are involved.[25]
ELISA and microscopic agglutination tests are virtually commonly used to diagnose leptospirosis in animals. The bacteria can be detected in claret, urine, and milk or liver, kidney, or other tissue samples past using immunofluorescence or immunohistochemical or polymerase chain reaction techniques. Silver staining or immunogold silver staining is used to detect Leptospira in tissue sections. The organisms stain poorly with Gram stain. Dark-field microscopy tin be used to discover Leptospira in torso fluids, merely it is neither sensitive nor specific in detecting the organism. A positive culture for leptospirosis is definitive, but the availability is limited, and culture results tin take 13–26 weeks for a result, limiting its utility. Paired astute and ambulatory samples are preferred for serological diagnosis of leptospirosis in animals. A positive serological sample from an aborted fetus is also diagnostic of leptospirosis.[25]
Various antibiotics such as doxycycline, penicillins, dihydrostreptomycin, and streptomycin have been used to treat leptospirosis in animals. Fluid therapy, blood transfusion, and respiratory support may exist required in severe disease. For horses with ERU, the main treatment is with anti-inflammatory drugs.[25] [15]
Leptospirosis vaccines are available for animals such as pigs, dogs, cattle, sheep, and goats. Vaccines for cattle usually contain Leptospira serovar Hardjo and Pomona, for dogs, the vaccines commonly incorporate serovar Icterohaemorrhagiae and Canicola. Vaccines containing multiple serovars practise not work for cattle every bit well as vaccines containing a single serovar, yet the multivalent vaccines continue to be sold.[15] Isolation of infected animals and rubber antibiotics are also constructive in preventing leptospirosis transmission between animals. Ecology control and sanitation likewise reduce transmission rates.[25] [fifteen]
References [edit]
- ^ Berger S (2018). Leptospirosis: Global Status. GIDEON Computer science Inc. p. 7. ISBN9781498820318.
- ^ Mosby's Medical Dictionary (9 ed.). Elsevier Health Sciences. 2013. p. 697. ISBN9780323112581. Archived from the original on 8 September 2017. Retrieved 21 February 2016.
- ^ McKay JE (2001). Comprehensive Health Care for Dogs. Minnetonka, MN.: Creative Pub. International. p. 97. ISBN9781559717830.
- ^ James WD, Elston DM, Berger TG, Andrews GC (2006). Andrews' Diseases of the Peel: Clinical Dermatology. Saunders Elsevier. ISBN978-0-7216-2921-vi. : 290
- ^ a b c d east f thou h i j thou l chiliad due north o Soo ZM, Khan NA, Siddiqui R (January 2020). "Leptospirosis: Increasing importance in developing countries". Acta Tropica. 201: 105183. doi:10.1016/j.actatropica.2019.105183. PMID 31542372.
- ^ a b McBride AJ, Athanazio DA, Reis MG, Ko AI (October 2005). "Leptospirosis". Electric current Opinion in Infectious Diseases. 18 (5): 376–86. doi:x.1097/01.qco.0000178824.05715.2c. PMID 16148523. S2CID 220576544.
- ^ a b c d e f g h i j yard l m n o p q r south t u v w x Karpagam KB, Ganesh B (January 2020). "Leptospirosis: a neglected tropical zoonotic infection of public health importance-an updated review". European Journal of Clinical Microbiology & Infectious Diseases. 39 (five): 835–846. doi:10.1007/s10096-019-03797-4. PMID 31898795. S2CID 209669669.
- ^ a b c d e f g h i j thousand fifty m due north o p q r s t u v w x y z aa ab ac ad ae af ag Lane, Alison B; Dore, Michael K (2016). "Leptospirosis: A clinical review of testify based diagnosis, treatment and prevention". World Journal of Clinical Infectious Diseases. 6 (4): 61. doi:10.5495/wjcid.v6.i4.61. ISSN 2220-3176.
- ^ Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White NJ (2013). Manson's Tropical Diseases E-Volume. Elsevier Health Sciences. p. 438. ISBN9780702053061. Archived from the original on eight September 2017. Retrieved 2 September 2017.
- ^ a b c d Evangelista KV, Coburn J (September 2010). "Leptospira as an emerging pathogen: a review of its biological science, pathogenesis and host allowed responses". Future Microbiology. v (9): 1413–25. doi:x.2217/fmb.10.102. PMC3037011. PMID 20860485.
- ^ a b c d Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. (2015). "Global Morbidity and Mortality of Leptospirosis: A Systematic Review". PLOS Neglected Tropical Diseases. 9 (nine): e0003898. doi:10.1371/journal.pntd.0003898. PMC4574773. PMID 26379143.
- ^ a b c d e f g h i j k l Picardeau G (May 2017). "Virulence of the zoonotic amanuensis of leptospirosis: still terra incognita?". Nature Reviews. Microbiology. 15 (5): 297–307. doi:10.1038/nrmicro.2017.v. PMID 28260786. S2CID 11626842.
- ^ Chan, O. Y.; Chia, S. E.; Nadarajah, North.; Sng, E. H. (xvi October 1987). "Leptospirosis Risk in Public Cleansing and Sewer Workers". Annals of the University of Medicine, Singapore. xvi (iv): 586–90. PMID 3446001.
- ^ a b c Teixeira AF, Fernandes LG, Cavenague MF, Takahashi MB, Santos JC, Passalia FJ, et al. (July 2019). "Adjuvanted leptospiral vaccines: Challenges and future development of new leptospirosis vaccines". Vaccine. 37 (30): 3961–3973. doi:10.1016/j.vaccine.2019.05.087. PMID 31186193. S2CID 186204949.
- ^ a b c d e f g h i j k 50 Ellis WA (2015). "Creature leptospirosis". Electric current Topics in Microbiology and Immunology. 387: 99–137. doi:10.1007/978-3-662-45059-8_6. ISBN978-3-662-45058-1. PMID 25388134.
- ^ Slack A (July 2010). "Leptospirosis". Australian Family unit Physician. 39 (7): 495–8. PMID 20628664.
- ^ a b Weil A (1886). "Über eine eigenthümliche, mit Milztumor, Icterus und Nephritis einhergehende, acute Infektionskrankheit" [On a strange, astute communicable diseases, accompanied by swelling of the spleen, icterus, and nephritis]. Deutsches Archiv für Klinische Medizin (in German). 39: 209–232.
- ^ a b c d e f k h i j k l 1000 northward o p q r south t u v w x y z aa ab ac advertising ae af ag ah ai aj ak al am an ao ap Haake DA, Levett PN (25 May 2015). "Leptospirosis in humans". Current Topics in Microbiology and Immunology. 387 (387): 65–97. doi:10.1007/978-three-662-45059-8_5. ISBN978-iii-662-45058-1. PMC4442676. PMID 25388133.
- ^ "Factsheet about leptospirosis". European Heart for Disease Prevention and Control . Retrieved 5 September 2020.
- ^ Waggoner JJ, Pinsky BA (October 2016). "Molecular diagnostics for homo leptospirosis". Current Stance in Infectious Diseases. 29 (five): 440–5. doi:10.1097/QCO.0000000000000295. PMC5127924. PMID 27537829.
- ^ a b Cagliero J, Villanueva SY, Matsui M (20 June 2018). "Leptospirosis Pathophysiology: Into the Storm of Cytokines". Frontiers in Cellular and Infection Microbiology. 8 (204): 204. doi:x.3389/fcimb.2018.00204. PMC6019470. PMID 29974037.
- ^ a b c Bennett JE, Raphael D, Martin JB, Bart JC (2015). "223". Mandell, Douglas, and Bennett'south Principles and Practice of Infectious Diseases (Eighth ed.). Elsevier. pp. 2541–2549. ISBN978-one-4557-4801-3.
- ^ Cameron CE (2015). "Leptospiral structure, physiology, and metabolism". Current Topics in Microbiology and Immunology. 387: 21–41. doi:10.1007/978-three-662-45059-8_3. ISBN978-3-662-45058-1. PMID 25388131.
- ^ a b c Caimi K, Ruybal P (Feb 2020). "Leptospira spp., a genus in the stage of diversity and genomic data expansion". Infection, Genetics and Evolution. 81: 104241. doi:ten.1016/j.meegid.2020.104241. PMID 32061688. S2CID 211135356.
- ^ a b c d e f g h i j k 50 m Spickler AR, Leedom Larson KR (Oct 2013). "Leptospirosis (Fact sail)" (PDF). The Center for Food Security and Public Health. Archived (PDF) from the original on 24 November 2014. Retrieved fifteen March 2019.
- ^ Barragan V, Olivas S, Keim P, Pearson T (October 2017). "Critical Knowledge Gaps in Our Understanding of Environmental Cycling and Manual of Leptospira spp". Applied and Environmental Microbiology. 83 (19). Bibcode:2017ApEnM..83E1190B. doi:ten.1128/AEM.01190-17. PMC5601346. PMID 28754706.
- ^ Allan KJ, Biggs HM, Halliday JE, Kazwala RR, Maro VP, Cleaveland S, Crump JA (2015). "Epidemiology of Leptospirosis in Africa: A Systematic Review of a Neglected Zoonosis and a Paradigm for '1 Health' in Africa". PLOS Neglected Tropical Diseases. 9 (9): e0003899. doi:10.1371/journal.pntd.0003899. PMC4569256. PMID 26368568.
- ^ a b c d e Chin VK, Basir R, Nordin SA, Abdullah 1000, Sekawi Z (March 2019). "Pathology and Host Immune Evasion During Human Leptospirosis: a Review" (PDF). International Microbiology. 23 (2): 127–136. doi:10.1007/s10123-019-00067-iii. PMID 30875033. S2CID 78095369.
- ^ Song AT, Abas L, Andrade LC, Andraus W, D'Albuquerque LA, Abdala Eastward (February 2016). "A first report of leptospirosis after liver transplantation". Transplant Infectious Disease. 18 (1): 137–40. doi:10.1111/tid.12490. PMID 26671230. S2CID 3548455.
- ^ Puliyath G, Singh S (October 2012). "Leptospirosis in pregnancy". European Journal of Clinical Microbiology & Infectious Diseases. 31 (x): 2491–6. doi:10.1007/s10096-012-1625-vii. PMID 22549729. S2CID 14033595.
- ^ Carles M, Montoya E, Joly F, Peneau C (1995). "[Leptospirosis and pregnancy. 11 cases in French Guyana]". Journal de Gynécologie, Obstétrique et Biologie de la Reproduction. 24 (iv): 418–21. PMID 7650320.
- ^ Koe SL, Tan KT, Tan TC (February 2014). "Leptospirosis in pregnancy with pathological fetal cardiotocography changes". Singapore Medical Journal. 55 (two): e20-4. doi:x.11622/smedj.2013194. PMC4291937. PMID 24712035.
- ^ Shaked Y, Shpilberg O, Samra D, Samra Y (August 1993). "Leptospirosis in pregnancy and its effect on the fetus: example report and review". Clinical Infectious Diseases. 17 (2): 241–3. doi:x.1093/clinids/17.two.241. PMID 8399874.
- ^ Fabiani, Adam; Dal Bo, Eugenia; Di Bella, Stefano; Gabrielli, Marco; Bologna, Alessandro; Albert, Umberto; Sanson, Gianfranco (5 July 2021). "Pica (Allotriophagy): An Underestimated Run a risk Gene for Severe Leptospirosis (Weil's Diseases)? Report of a Leptospira Septic Shock Successfully Managed with ECMO". Communicable diseases Reports. 13 (3): 619–626. doi:10.3390/idr13030058. ISSN 2036-7449. PMC8293114. PMID 34287302.
- ^ Kumar SS (2013). "7" (PDF). Indian Guidelines for the Diagnosis and Management of Human being Leptospirosis. India. pp. 23–29. Archived from the original (PDF) on 25 Dec 2016. Retrieved xvi November 2019.
- ^ a b c Xu Y, Ye Q (April 2018). "Human leptospirosis vaccines in China". Human Vaccines & Immunotherapeutics. 14 (iv): 984–993. doi:ten.1080/21645515.2017.1405884. PMC5893195. PMID 29148958.
- ^ Abd Rahim MA, Zaki AM, Atil A, Azme MH, Him NA, Rahim SS, Jeffree MS, Ahmad N, Hassan MR. "Effectiveness of Antibiotic Prophylaxis for Leptospirosis among Adults: A Systematic Review". Malaysian Periodical of Applied Sciences. iii (2): 46–56. Retrieved 1 March 2020.
- ^ a b WHO recommended strategies for the prevention and command of catching diseases. World Health Organization – Department of Communicable Disease Control, Prevention and Eradication. 2001. p. 104. Archived from the original on five May 2019.
- ^ Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W (June 2003). "Ceftriaxone compared with sodium penicillin thou for treatment of astringent leptospirosis". Clinical Infectious Diseases. 36 (12): 1507–13. doi:ten.1086/375226. PMID 12802748.
- ^ Suputtamongkol Y, Niwattayakul G, Suttinont C, Losuwanaluk K, Limpaiboon R, Chierakul West, et al. (November 2004). "An open up, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis". Clinical Infectious Diseases. 39 (10): 1417–24. doi:10.1086/425001. PMID 15546074.
- ^ Phimda Grand, Hoontrakul S, Suttinont C, Chareonwat S, Losuwanaluk K, Chueasuwanchai S, et al. (September 2007). "Doxycycline versus azithromycin for handling of leptospirosis and scrub typhus". Antimicrobial Agents and Chemotherapy. 51 (9): 3259–63. doi:10.1128/AAC.00508-07. PMC2043199. PMID 17638700.
- ^ a b Brett-Major DM, Coldren R (February 2012). "Antibiotics for leptospirosis". The Cochrane Database of Systematic Reviews (2): CD008264. doi:10.1002/14651858.CD008264.pub2. PMID 22336839.
- ^ a b Charan J, Saxena D, Mulla South, Yadav P (May 2013). "Antibiotics for the treatment of leptospirosis: systematic review and meta-analysis of controlled trials". International Journal of Preventive Medicine. 4 (5): 501–10. PMC3733179. PMID 23930159.
- ^ Rodrigo C, Lakshitha de Silva Due north, Goonaratne R, Samarasekara K, Wijesinghe I, Parththipan B, Rajapakse S (Dec 2014). "High dose corticosteroids in astringent leptospirosis: a systematic review". Transactions of the Royal Guild of Tropical Medicine and Hygiene. 108 (12): 743–50. doi:10.1093/trstmh/tru148. PMID 25266477.
- ^ a b "Healthcare Workers – Technical Information for Leptospirosis". Centers for Affliction Control and Prevention (CDC). 9 Nov 2017. Archived from the original on 11 Jan 2019. Retrieved 28 April 2019.
- ^ Guerra MA (September 2013). "Leptospirosis: public health perspectives". Biologicals. 41 (v): 295–seven. doi:10.1016/j.biologicals.2013.06.010. PMC4629849. PMID 23850378.
- ^ Moola, Sandeep; Beri, Deepti; Salam, Abdul; Jagnoor, Jagnoor; Teja, Arun; Bhaumik, Soumyadeep (July 2021). "Leptospirosis prevalence and risk factors in Bharat: Evidence gap maps". Tropical Md. 51 (3): 415–421. doi:10.1177/00494755211005203. ISSN 1758-1133. PMID 33832378. S2CID 233191847.
- ^ "WHO | Leptospirosis Brunt Epidemiology Reference Group (LERG)". world wide web.who.int. Archived from the original on 17 November 2017. Retrieved 30 Nov 2017.
- ^ a b Herman HS, Mehta S, Cárdenas WB, Stewart-Ibarra AM, Finkelstein JL (July 2016). "Micronutrients and Leptospirosis: A Review of the Electric current Evidence". PLOS Neglected Tropical Diseases. 10 (7): e0004652. doi:10.1371/journal.pntd.0004652. PMC4936698. PMID 27387046.
- ^ a b c d eastward f thousand h i j m Adler B (2015). "History of leptospirosis and leptospira". Electric current Topics in Microbiology and Immunology. 387: 1–nine. doi:x.1007/978-3-662-45059-8_1. ISBN978-3-662-45058-one. PMID 25388129.
- ^ Dorland's illustrated medical dictionary. Philadelphia: Elsevier/Saunders. 2012. p. 1231. ISBN9781455709854. Archived from the original on 8 September 2017. Retrieved 21 Feb 2016.
- ^ Clapham D (2004). Pocket-size H2o Supplies: A Practical Guide. Routledge. p. 125. ISBN9781134457496. Archived from the original on 8 September 2017. Retrieved 21 Feb 2016.
- ^ Christmas BW, Tennent RB, Lindsay PG (May 1974). "Dairy farm fever in New Zealand: a local outbreak of human leptospirosis". The New Zealand Medical Periodical. 79 (514): 901–4. PMID 4527727.
- ^ Marr JS, Cathey JT (Feb 2010). "New hypothesis for crusade of epidemic amongst native Americans, New England, 1616-1619". Emerging Infectious Diseases. 16 (2): 281–6. doi:10.3201/eid1602.090276. PMC2957993. PMID 20113559.
- ^ Stimson AM (1907). "Note on an organism found in yellow-fever tissue". Public Health Reports. 22 (18): 541. doi:x.2307/4559008. JSTOR 4559008.
- ^ Inada R, Ito Y (1908). "A report of the discovery of the causal organism (a new species of spirocheta) of Weil'south affliction". Tokyo Ijishinshi. 1915: 351–60.
- ^ Inada R, Ido Y, Hoki R, Kaneko R, Ito H (March 1916). "The Etiology, Mode of Infection, and Specific Therapy of Weil's Disease (Spirochætosis Icterohæmorrhagica)". The Journal of Experimental Medicine. 23 (3): 377–402. doi:10.1084/jem.23.three.377. PMC2125418. PMID 19867994.
- ^ Paniz-Mondolfi AE, Rodriguez-Morales AJ, Blohm G, Marquez M, Villamil-Gomez WE (July 2016). "ChikDenMaZika Syndrome: the challenge of diagnosing arboviral infections in the midst of concurrent epidemics". Register of Clinical Microbiology and Antimicrobials. 15 (ane): 42. doi:10.1186/s12941-016-0157-x. PMC4957883. PMID 27449770.
- ^ "284184004: Lepthangamushi syndrome (disorder)". Archived from the original on 18 November 2019. Retrieved 18 November 2019.
- ^ a b "Leptospirosis" (PDF). The Center for Food Security and Public Health. October 2013. Archived (PDF) from the original on 24 November 2014. Retrieved 8 November 2014.
- ^ Klopfleisch R, Kohn B, Plog S, Weingart C, Nöckler K, Mayer-Scholl A, Gruber AD (December 2010). "An emerging pulmonary haemorrhagic syndrome in dogs: similar to the human leptospiral pulmonary haemorrhagic syndrome?". Veterinarian Medicine International. 2010: 928541. doi:10.4061/2010/928541. PMC3025382. PMID 21274452.
- ^ a b Zuerner RL (2015). "Host response to Leptospira infection". Current Topics in Microbiology and Immunology. 387: 223–l. doi:10.1007/978-3-662-45059-8_9. ISBN978-3-662-45058-1. PMID 25388137.
- ^ a b Divers TJ, Chang YF, Irby NL, Smith JL, Carter CN (May 2019). "Leptospirosis: An important communicable diseases in N American horses". Equine Veterinary Periodical. 51 (3): 287–292. doi:10.1111/evj.13069. PMID 30629756. S2CID 58578433.
External links [edit]
- "Leptospirosis". U.S. Illness Control and Prevention Center. 21 November 2018.
- "Leptospira". NCBI Taxonomy Browser. 171.
Source: https://en.wikipedia.org/wiki/Leptospirosis
Posted by: robertslethed.blogspot.com

0 Response to "Which Animal Bite May Pose A Risk For Rabies?"
Post a Comment